This post continues our series highlighting the value of 7-1-7 bottleneck analysis and exploring solutions to the top barriers to outbreak detection, reporting and response.
Previous posts in this series examined bottleneck trends across 500 bottlenecks from 148 outbreaks in 18 partner countries and explored solutions to resource barriers and low clinical suspicion. In this post, we dive into delays in laboratory confirmation, which can derail the outbreak response.
What the data showed and why it matters
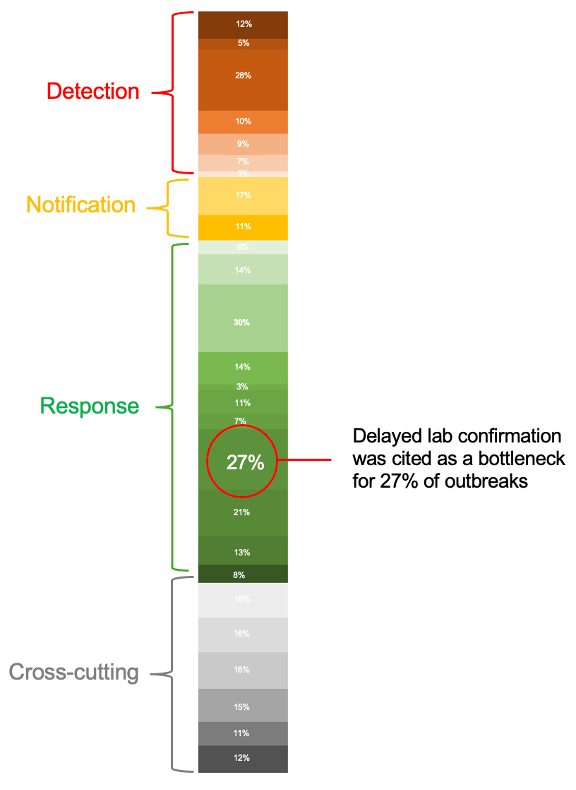
Above: Bottleneck synthesis showing how the 500 bottlenecks are distributed across 26 categories and four outbreak phases – see the full bottleneck synthesis in our previous post
Timely diagnostic testing is crucial to identify the pathogen causing an outbreak and stop it early.
Getting rapid laboratory confirmation during an outbreak investigation requires multiple steps to work together smoothly. Health officials need to coordinate the collection of a specimen and its transport to a laboratory. That lab needs to have staff, capacity and resources to conduct tests in a safe environment. Then, the laboratory must quickly communicate the results back to officials to guide outbreak response.
In our analysis, delayed laboratory confirmation was reported in 27% of outbreaks—making it one of the most common bottlenecks encountered during the response, second only to inadequate resources for early response.
Below: Lab confirmation was affected by connected bottlenecks upstream, including delayed specimen collection and transportation and lack of diagnostics commodities.
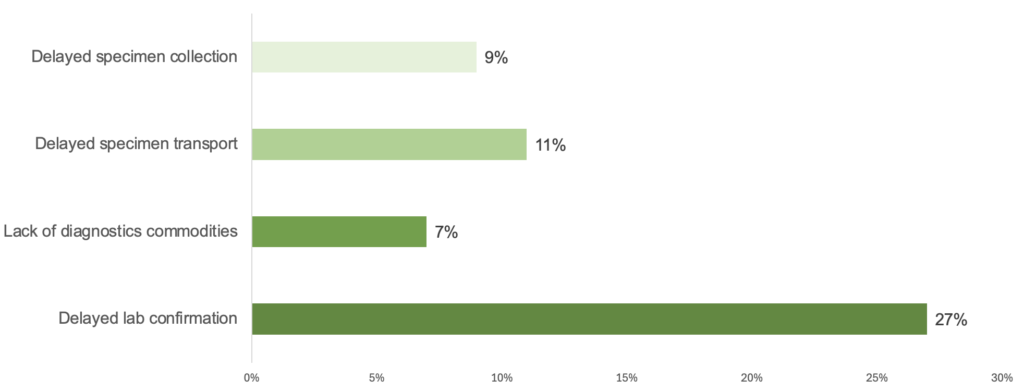
Delays in laboratory confirmation were often connected to delayed specimen collection (9% of outbreaks) and transportation (11%) and a lack of diagnostic commodities, such as tests and reagents needed for laboratory testing (7%). To accelerate laboratory confirmation, countries need to address the full diagnostic chain.
Delayed lab confirmation can quickly derail outbreak response because other 7-1-7 early response actions depend on timeliness. Knowledge of the pathogen is critical for appropriate case management and infection prevention and control, risk communication and community engagement, public health and social measures, and for establishing an appropriate coordination mechanism.
In the 7-1-7 target, is lab confirmation part of detection or early response?
Lab confirmation is one of the seven 7-1-7 early response actions; the date of detection can be based solely on suspicion of the event before laboratory testing happens. To meet the target, lab confirmation must occur no more than seven days after public health authorities receive notification of the suspected outbreak[1]. Because some lab confirmation protocols use tests based on a person’s immune response to an infection over several weeks, lab confirmation may not be possible within the target. To learn how to determine dates to calculate 7-1-7 performance, refer to the 7-1-7 Milestones Reference Guide.
[1] Specific outbreaks may require a confirmed case to become a notifiable event (e.g., human influenza caused by a new sub-type). For these outbreaks, laboratory confirmation may occur earlier or on the same date as the date of detection. If public health authorities first become aware of an event through a laboratory result, the date they received the result is the date of notification.
How a lack of diagnostics stoked fears in the Democratic Republic of Congo and globally
A 2024 outbreak in the Democratic Republic of Congo highlights the critical role of timely diagnostics in confirming an outbreak and directing the response.
On November 29, 2024, local health authorities in Kwango Province, DRC reported an undiagnosed outbreak. By mid-December, 891 persons had become ill with an unknown illness, and 48 deaths were reported, with young children disproportionately affected.
Laboratory confirmation was delayed due to limited testing capacity in the region, including a lack of laboratory staff and reagents. The absence of a diagnosis and high number of deaths sparked fears that the unknown disease could spread across the country and internationally.
On December 16, two weeks after the outbreak was first reported, laboratory results from 430 samples were finally communicated. They showed positive results for malaria and common respiratory viruses. Multidisciplinary rapid response teams were then deployed to investigate the event and strengthen the response. The teams provided support for diagnosis, the treatment of patients, risk communication and community engagement.
What it means for countries
Plan ahead
Because the burden and distribution of infectious diseases vary widely from country to country, there isn’t a one-size-fits-all solution. Countries should plan for laboratory confirmation of outbreaks based on local risks and design strategies to ensure that testing can be conducted promptly, whenever required.
Tools such as WHO’s National Essential Diagnostics Lists (NEDL) help identify which diagnostics should be available at each health system level. While developing NEDLs, countries should include diagnostics for the detection and confirmation of disease outbreaks based on:
- their risk of occurrence
- the different levels of the system – from pathogen genomic sequencing in central or public health laboratories to point-of-care and near point-of-care tests used at a patient’s bedside.
This planning will assist countries’ efforts to address regulatory, market access and technical capacity barriers to deploy diagnostics for disease outbreaks.
Strengthen testing capacity
Countries can strengthen testing capacity and quality management systems within individual public health laboratories or designated clinical laboratories using these tools:
- Assess individual labs and networks: Countries can assess the performance of individual laboratories using the WHO Laboratory Assessment Tool.
- Optimize laboratory networks: Where a is used to detect and respond to outbreaks, countries can assess the functionality of their with support from Resolve to Save Lives. In addition, countries can perform diagnostic network optimization – an approach developed by the African Society for Laboratory Medicine (ASLM) and FIND that analyzes geospatial and testing data to maximize access to diagnostics. Recommendations from this type of analysis include the procurement of new devices, the relocation of existing ones, adjustments to the sample transport system and the establishment of new labs.
Avoid unnecessary referrals
Timely lab confirmation is more likely when unnecessary referrals and transport of patient samples to distant laboratories are minimized. Health care workers and responders should be trained on case definitions for outbreaks to ensure samples are obtained only from patients that meet case criteria.
In addition, improving diagnosis of non-outbreak conditions – including using rapid or point of care tests – can reduce the burden on reference laboratories, allowing them to focus on urgent outbreak testing.
The laboratory confirmation bottleneck is complex, but 7-1-7 performance can be improved by planning for and acquiring the right diagnostic tools for outbreaks, improving the quality and availability of testing for outbreaks and by minimizing unnecessary testing.
How a 7-1-7 bottleneck analysis can help identify the root causes of delays in the diagnostic chain
Lab confirmation bottlenecks can be challenging. Understanding where the delay is in the diagnostic chain and coming up with a specific solution are critical. That’s where 7-1-7 can help. When applying 7-1-7 to an outbreak, countries look for the specific bottlenecks that hindered fast detection, notification and response to the outbreak.
A good bottleneck analysis requires knowing how to get to an actionable root cause in order to identify the right corrective actions. The ‘Five Whys’ approach is a simple method for moving beyond superficial bottlenecks and get more granular. “Why” is simply asked up to five times in succession until an actionable root cause is determined. Learn more about conducting a good bottleneck analysis in our toolkit.